Describe the Process in Compliance Reporting Conflicts of Interest
Informed consent is the process of telling potential research particpants about the key elements of a research study and what their participation will involve. When submitting a grant application the signature of the Authorized Organization Representative AOR certifies the applicant institutions compliance with the requirements of 42 CFR 50 Subpart F including that.
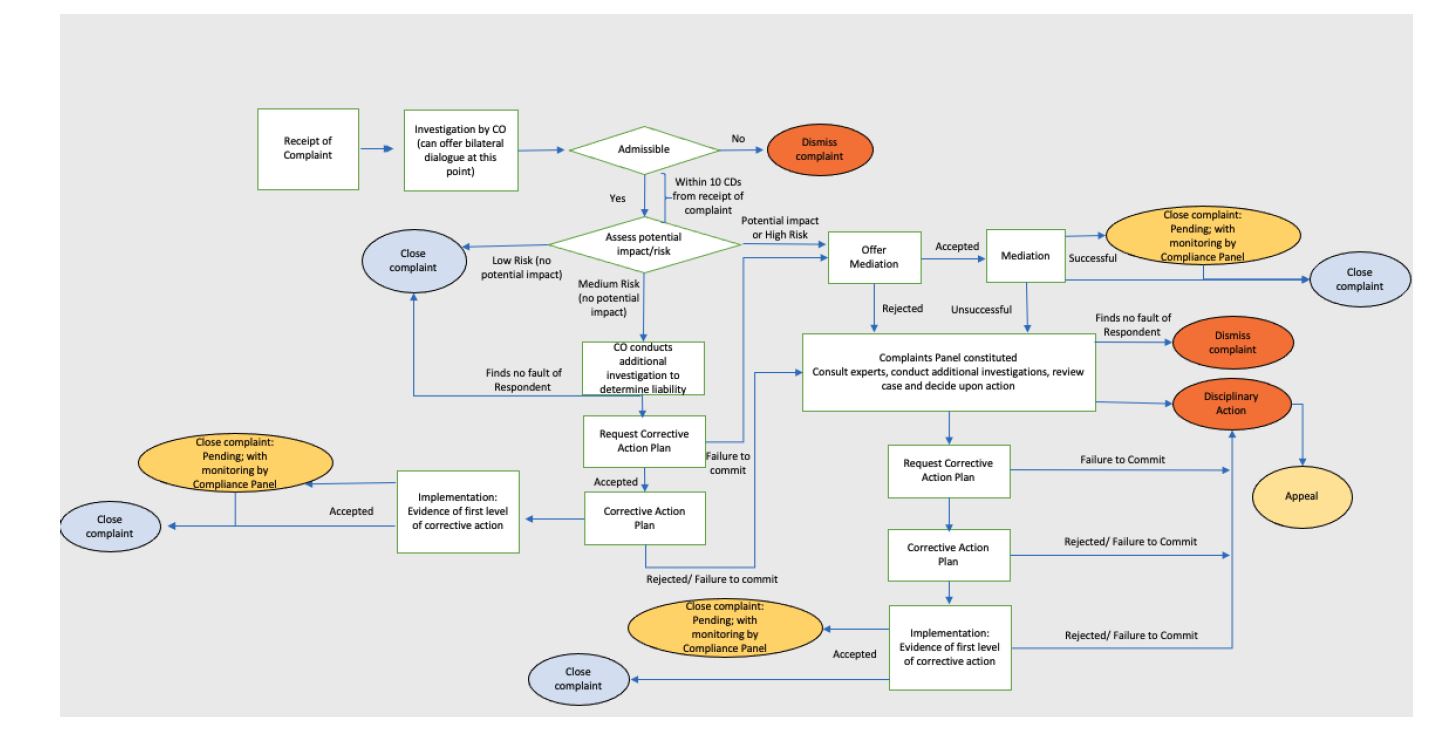
Grievance Procedure Global Platform For Sustainable Natural Rubber
The grants management policies on this page are applicable to all Executive Branch agencies boards.
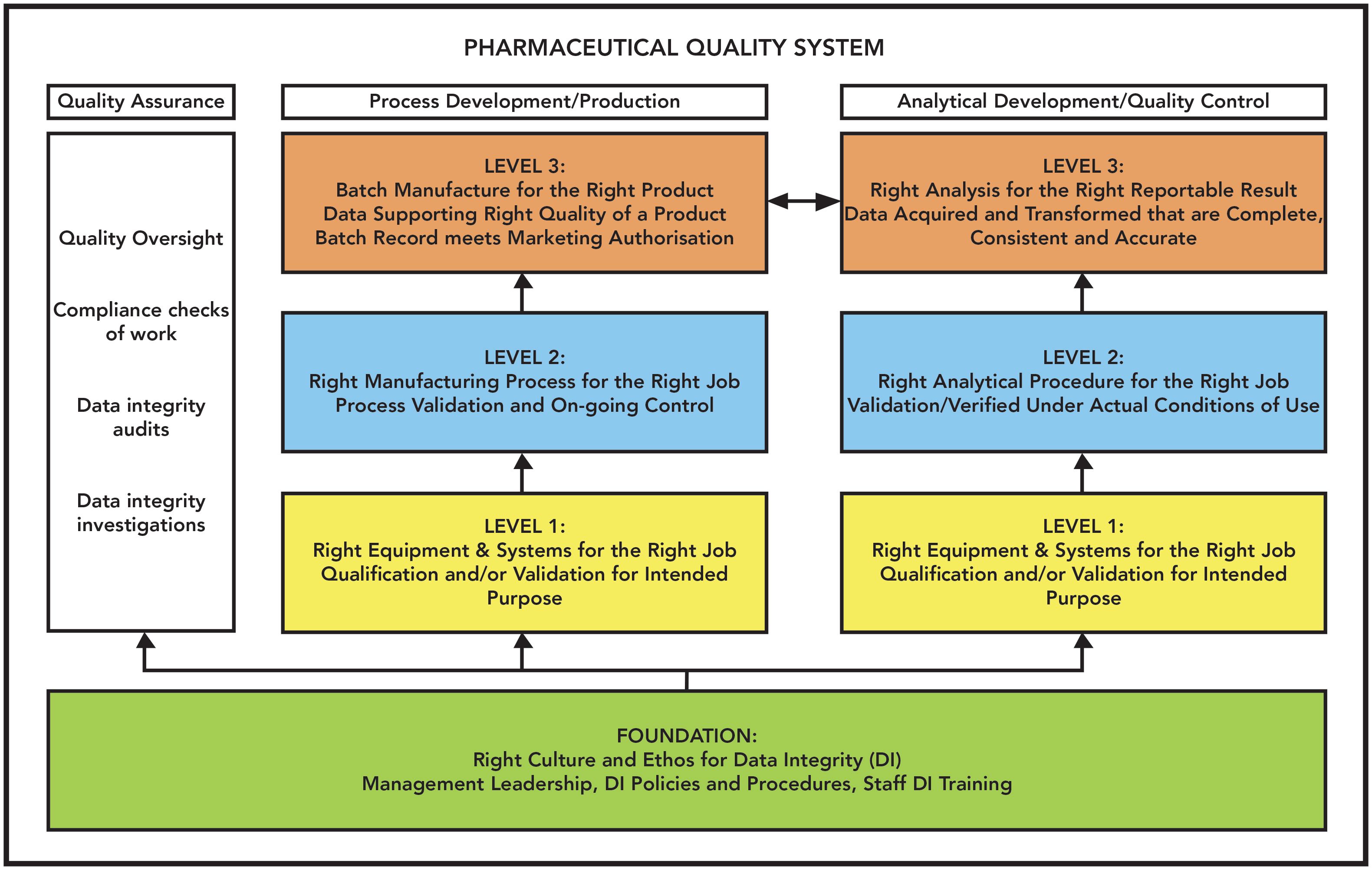
. It is the policy of the university not to hire any person convicted of a crime the nature of which is reasonably related to the applicants fitness for the job. Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research eg human subjects research export controls conflict of interest. Or 4 pursuant to our or a third partys legitimate interest provided such interests are not overridden by any of your.
They then describe a five-step process for identifying measuring and managing non-financial conflicts of interest. Regulatory Management for IRB or IBC rDNA applications. The term conflict of interest COI in research refers to situations in which financial or other personal considerations may compromise or have the appearance of compromising an investigators professional judgment in conducting or reporting research.
Improving the states grant-making is one of the primary goals of Admins Office of Grants Management. Lastly your organization should have tracking and reporting systems in place which help manage the conflicts of interest disclosure process. An appropriate system will facilitate communication between compliance officers and the disclosing employee.
NIH incorporates these notices into the annual update of the NIH Grants Policy Statement. NIH Grants Policy Statement - Dec 2021 HTML PDF Applies to all NIH grants and cooperative agreements with budget periods beginning on or after October 1 2021. Significant Changes since April.
1 with your consent. This part- a Gives instructions for using provisions and clauses in solicitations andor contracts. Being able to see at a glance who received.
Questions about the process or help troubleshooting can be directed to UW Human Resources at 307-766-2377. Compliance with these policy updates also becomes a term and condition of award. It is most applicable to teams such as systematic review teams and practice guideline development.
Federal agencies have their own set of rules governing financial conflicts of interest for employees. And c Presents a matrix listing the FAR provisions and clauses applicable to each principal contract type andor purpose eg fixed-price supply cost-reimbursement research and development. Generally we process your Personal Data.
Reporting and Automating Conflicts of Interest. The consent process typically includes providing a written consent document containing the required. No person convicted of a felony of any nature shall be hired without the approval of the hiring.
A COI depends on the situation and not on the actions or character of an individual investigator. 2 subject to the performance of a contract to which you as the data subject are a party. 3 in order to take steps at your request to enter into an agreement with Rethink Compliance.
The informed consent process is one of the central components of the ethical conduct of research with human subjects. One tool for accomplishing this is the creation of comprehensive grants management policies as outlined in Minnesota Statute 16B97 sub. B Sets forth the solicitation provisions and contract clauses prescribed by this regulation.
This process requires transparency in documentation accounts for context and relies on judgment in evaluating risk of particular conflicts. EResearch is U-Ms site for electronic research administration. Should be the starting point for all recipients and subrecipients of DOJ grants and cooperative agreements in ensuring the efective day-to-day management of awards.

Understand The Cost Of Noncompliance

Correctional Officer Resume With No Experience Luxury Unusual Correctional Ficer Resume Philaurbansolutions
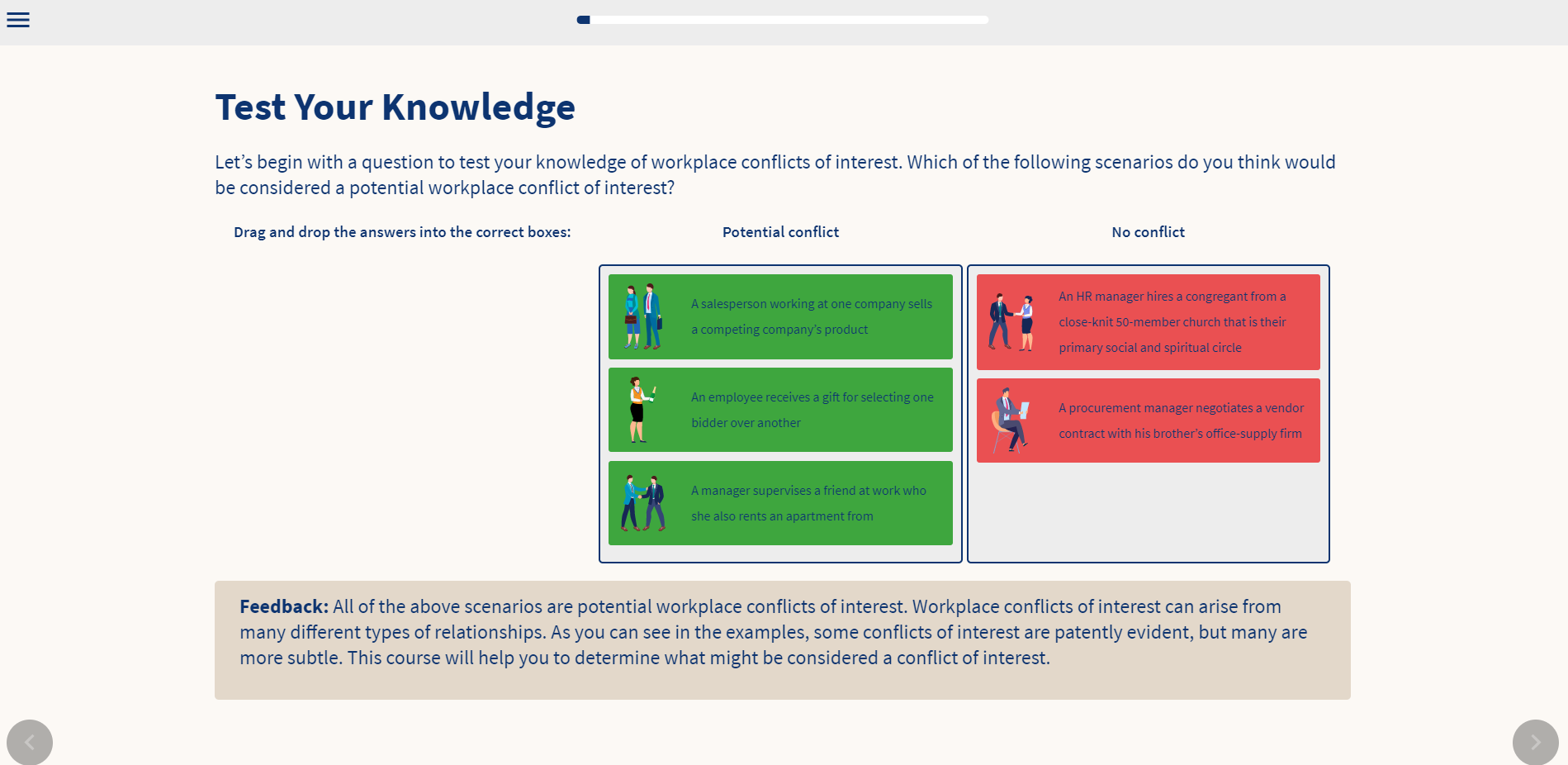
Conflict Of Interest At Work And Its Compliance Implications Vinciworks
No comments for "Describe the Process in Compliance Reporting Conflicts of Interest"
Post a Comment